Main Content
Single-cell activities of bacterial pathogens during infection and treatment
We study mechanism of bacterial infections in host tissues as a basis for novel control strategies.
Bacterial infections are a major threat to human health. New antibiotics and vaccines are urgently required but progress has been slow. A major barrier is our limited understanding of how bacteria change their properties in host tissues compared to the fundamentally different laboratory conditions that are used in most studies.
In vivo infection biology and treatment of Salmonella
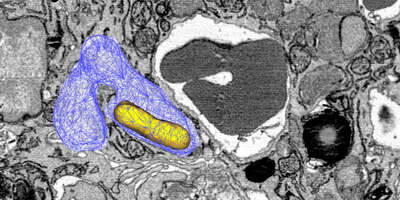
We focus on the following key questions: Where are the bacteria located within host tissues? How does the host control bacterial growth? How do bacteria adapt to their local microenvironments? And how do these interactions affect antimicrobial chemotherapy? To address these questions, we combine molecular biology, flow cytometry, mass spectrometry, and advanced imaging techniques such as 2-photon tomography of entire organs with micrometer resolution.
Recently, we identified severe magnesium deprivation as a critical defense mechanism against intracellular Salmonella. This nutrient limitation triggers slow bacterial growth, increasing their resilience against stresses including antibiotics. This impairs antibiotic clearance of Salmonella from tissues, particularly in tissue compartments with weak local inflammation. Additionally, we showed that standard plating assays to measure antibiotic killing can produce confounded and misleading results. For more accurate measurements, single-cell real-time assays are required.
Human infections
We are currently extending our research to include biopsies of patients infected with Staphylococcus aureus, Pseudomonas aeruginosa, and E. coli, in collaboration with the University Hospitals Basel and Zurich as part of the NCCR AntiResist initiative.